sodium thiosulfate and iodine titration
 An official website of the United States government. However, this approach is not cost effective and in lab practice it is much better to use iodate as a primary substance to standardize thiosulfate, and then standardize iodine solution against thiosulfate. This solution is used for neutralization of sodium arsenite, before it is titrated with iodine solution during iodine solution standardization. ; Asking for help, clarification, or responding to other answers. Recknagel S, Breitenbach M, Pautz J, Lck D. Anal Chim Acta. There are actually two chemical reactions going on at the same time when you combine the solutions. Then moles of iodine = 1.32 x 10 mol / 2 = 6.60 x 10 mol. anita baker first husband; sodium thiosulfate and iodine titration. forming a sulfate instead: $$\ce{S2O3^2- + 4 Cl2 + 5 H2O -> 2 HSO4^- + 8 H+ + 8 Cl-}$$. How much lactose is there in milk (mechanism)? This site needs JavaScript to work properly. The liberation process was discussed from the changes in the apparent assay of potassium iodate. When all the iodine has reacted with the thiosulphate solution, the dark blue colour will disappear. This lowers free iodine concentration and such solutions are stable enough to be used in lab practice. If the blue-black coloration is still visible, add more sodium thiosulfate. So, the end point of the titration is when the dark blue colour disappears. Which solution in the iodine clock reaction is regarded as the indicator? trailer
<<
/Size 48
/Info 12 0 R
/Root 15 0 R
/Prev 86225
/ID[<8bbaa884f3eadd3356ca229487d108ad><562b836152c5bd02d67bc782cb22b6b7>]
>>
startxref
0
%%EOF
15 0 obj
<<
/Pages 11 0 R
/Type /Catalog
/PageLabels 9 0 R
/StructTreeRoot null
/Metadata 13 0 R
/OCProperties << /D << /Order [ ] /RBGroups [ ] >> /OCGs [ 42 0 R ] >>
/PieceInfo << /MarkedPDF << /LastModified (D:20050304204038)>> >>
/LastModified (D:20050304204038)
/MarkInfo << /Marked true /LetterspaceFlags 0 >>
>>
endobj
46 0 obj
<< /S 107 /L 199 /Filter /FlateDecode /Length 47 0 R >>
stream
Asked 2 years, 11 months ago. WebWe do have a titration solution and indicator that can measure the amount of I 3-in the sample. For this use the stoichiometry of the equation: 2 moles of thiosulfate ions are used per mole of iodine (Ratio 2:1), Therefore, if moles of thiosulfate = 1.32 x 10 mol official website and that any information you provide is encrypted Sulfur is being oxidized from a $+2$ state to a $+2.5$ state. Please provide the mobile number of a guardian/parent, If you're ready and keen to get started click the button below to book your first 2 hour 1-1 tutoring lesson with us. 0000023955 00000 n
Add 10-20 grams of sodium thiosulfate pentahydrate, swirl, cap the container, and let stand for 10-15 minutes. FOIA 2012;28(6):601-5. doi: 10.2116/analsci.28.601. Iodine reacts directly, fast and quantitively with many organic and inorganic substances. sodium thiosulfate and iodine titration. 2. The measurement is based on the titration of iodine by sodium thiosulfate. WebThe appropriate iodine liberation conditions to titrate potassium bromate with sodium thiosulfate were clarified through examinations by electrochemical methods. Potassium iodate was assayed by gravimetric titration with the sodium thiosulfate solution under several iodine liberation conditions. For gravimetric titration, the results obtained for the effective purity of potassium dichromate were sufficiently close to its certified value to allow confirmation of the validity of the gravimetric titration was confirmed. Finally, an accurate determination method for bromate was first established with high accuracy and traceability to the SI by primary methods. Step 4: Calculate the concentration of oxidising agent. Introduction a new piece of precision volumetric glassware, the.
An official website of the United States government. However, this approach is not cost effective and in lab practice it is much better to use iodate as a primary substance to standardize thiosulfate, and then standardize iodine solution against thiosulfate. This solution is used for neutralization of sodium arsenite, before it is titrated with iodine solution during iodine solution standardization. ; Asking for help, clarification, or responding to other answers. Recknagel S, Breitenbach M, Pautz J, Lck D. Anal Chim Acta. There are actually two chemical reactions going on at the same time when you combine the solutions. Then moles of iodine = 1.32 x 10 mol / 2 = 6.60 x 10 mol. anita baker first husband; sodium thiosulfate and iodine titration. forming a sulfate instead: $$\ce{S2O3^2- + 4 Cl2 + 5 H2O -> 2 HSO4^- + 8 H+ + 8 Cl-}$$. How much lactose is there in milk (mechanism)? This site needs JavaScript to work properly. The liberation process was discussed from the changes in the apparent assay of potassium iodate. When all the iodine has reacted with the thiosulphate solution, the dark blue colour will disappear. This lowers free iodine concentration and such solutions are stable enough to be used in lab practice. If the blue-black coloration is still visible, add more sodium thiosulfate. So, the end point of the titration is when the dark blue colour disappears. Which solution in the iodine clock reaction is regarded as the indicator? trailer
<<
/Size 48
/Info 12 0 R
/Root 15 0 R
/Prev 86225
/ID[<8bbaa884f3eadd3356ca229487d108ad><562b836152c5bd02d67bc782cb22b6b7>]
>>
startxref
0
%%EOF
15 0 obj
<<
/Pages 11 0 R
/Type /Catalog
/PageLabels 9 0 R
/StructTreeRoot null
/Metadata 13 0 R
/OCProperties << /D << /Order [ ] /RBGroups [ ] >> /OCGs [ 42 0 R ] >>
/PieceInfo << /MarkedPDF << /LastModified (D:20050304204038)>> >>
/LastModified (D:20050304204038)
/MarkInfo << /Marked true /LetterspaceFlags 0 >>
>>
endobj
46 0 obj
<< /S 107 /L 199 /Filter /FlateDecode /Length 47 0 R >>
stream
Asked 2 years, 11 months ago. WebWe do have a titration solution and indicator that can measure the amount of I 3-in the sample. For this use the stoichiometry of the equation: 2 moles of thiosulfate ions are used per mole of iodine (Ratio 2:1), Therefore, if moles of thiosulfate = 1.32 x 10 mol official website and that any information you provide is encrypted Sulfur is being oxidized from a $+2$ state to a $+2.5$ state. Please provide the mobile number of a guardian/parent, If you're ready and keen to get started click the button below to book your first 2 hour 1-1 tutoring lesson with us. 0000023955 00000 n
Add 10-20 grams of sodium thiosulfate pentahydrate, swirl, cap the container, and let stand for 10-15 minutes. FOIA 2012;28(6):601-5. doi: 10.2116/analsci.28.601. Iodine reacts directly, fast and quantitively with many organic and inorganic substances. sodium thiosulfate and iodine titration. 2. The measurement is based on the titration of iodine by sodium thiosulfate. WebThe appropriate iodine liberation conditions to titrate potassium bromate with sodium thiosulfate were clarified through examinations by electrochemical methods. Potassium iodate was assayed by gravimetric titration with the sodium thiosulfate solution under several iodine liberation conditions. For gravimetric titration, the results obtained for the effective purity of potassium dichromate were sufficiently close to its certified value to allow confirmation of the validity of the gravimetric titration was confirmed. Finally, an accurate determination method for bromate was first established with high accuracy and traceability to the SI by primary methods. Step 4: Calculate the concentration of oxidising agent. Introduction a new piece of precision volumetric glassware, the.  5.6.2 Run in replicate. The mixture should now appear murky white. It is routinely used as a titrant to determine concentrations of oxidants such as hypochlorite in bleach and dissolved oxygen in water. In order to find out the concentration of an oxidising agent, Iodine-Sodium Thiosulfate titrations can be used. Disponibles con pantallas touch, banda transportadora, brazo mecanico. The information of the appropriate titration procedure obtained in the present study is useful for any analysts utilizing potassium iodate to standardize a thiosulfate solution. 1. You will be provided with the following solutions: 0.2M potassium iodide, KI; (iv) 0.2M potassium chloride, KCl; (v) 0.1M potassium sulfate, K2SO4. Worked example: A student adds 25.0 cm of potassium iodate (V) solution to an excess of acidified potassium iodide solution. Podeli na Fejsbuku. Sodium bisulfite can be used to eliminate excess iodine. And why only sodium thiosulphate is used for titration? Accessibility 4 What happens when iodine is mixed with thiosulfate? Bethesda, MD 20894, Web Policies The best answers are voted up and rise to the top, Not the answer you're looking for? Epub 2007 Aug 2. It instantly dechlorinates water, and is used to stop bleaching action in the paper-making industry. Luke 23:44-48. Architektw 1405-270 MarkiPoland, free trial version of the concentration calculator, end point detection in iodometric titration, Solutions used in iodometric titrations. Precise coulometric titration of sodium thiosulfate and development of potassium iodate as a redox standard. of 1 per cent starch solution is added and the titration continued until the almost black color begins to turn a purple.
5.6.2 Run in replicate. The mixture should now appear murky white. It is routinely used as a titrant to determine concentrations of oxidants such as hypochlorite in bleach and dissolved oxygen in water. In order to find out the concentration of an oxidising agent, Iodine-Sodium Thiosulfate titrations can be used. Disponibles con pantallas touch, banda transportadora, brazo mecanico. The information of the appropriate titration procedure obtained in the present study is useful for any analysts utilizing potassium iodate to standardize a thiosulfate solution. 1. You will be provided with the following solutions: 0.2M potassium iodide, KI; (iv) 0.2M potassium chloride, KCl; (v) 0.1M potassium sulfate, K2SO4. Worked example: A student adds 25.0 cm of potassium iodate (V) solution to an excess of acidified potassium iodide solution. Podeli na Fejsbuku. Sodium bisulfite can be used to eliminate excess iodine. And why only sodium thiosulphate is used for titration? Accessibility 4 What happens when iodine is mixed with thiosulfate? Bethesda, MD 20894, Web Policies The best answers are voted up and rise to the top, Not the answer you're looking for? Epub 2007 Aug 2. It instantly dechlorinates water, and is used to stop bleaching action in the paper-making industry. Luke 23:44-48. Architektw 1405-270 MarkiPoland, free trial version of the concentration calculator, end point detection in iodometric titration, Solutions used in iodometric titrations. Precise coulometric titration of sodium thiosulfate and development of potassium iodate as a redox standard. of 1 per cent starch solution is added and the titration continued until the almost black color begins to turn a purple.  The steps involved in an Iodine-Sodium Thiosulfate Titration are: 1. WebSo, sodium carbonate can be used either to stabilize thiosulfate, or to lower its reaction rate with anything else that it's been combined with. Bookshelf Why is sodium thiosulfate used in iodometric titration? Would you like email updates of new search results? 3. 714-717]: $$\ce{S4O6^2- + 2 e- <=> 2 S2O3^2-} \qquad E^\circ = \pu{0.169 V}$$ Sodium thiosulfate is often standardized with potassium dichromate. Iodine is very weakly soluble in the water, and can be easily lost from the solution due to its volatility. _mhFH6!SIIB4*\}u.l|!8[sfpf6 They have unique physical and chemical properties that make them useful in various industries and applications.
The steps involved in an Iodine-Sodium Thiosulfate Titration are: 1. WebSo, sodium carbonate can be used either to stabilize thiosulfate, or to lower its reaction rate with anything else that it's been combined with. Bookshelf Why is sodium thiosulfate used in iodometric titration? Would you like email updates of new search results? 3. 714-717]: $$\ce{S4O6^2- + 2 e- <=> 2 S2O3^2-} \qquad E^\circ = \pu{0.169 V}$$ Sodium thiosulfate is often standardized with potassium dichromate. Iodine is very weakly soluble in the water, and can be easily lost from the solution due to its volatility. _mhFH6!SIIB4*\}u.l|!8[sfpf6 They have unique physical and chemical properties that make them useful in various industries and applications.  When starch and iodine are both present the solution is? Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. Coulometric titration was utilized to determine the concentration of a sodium thiosulfate solution on an absolute basis. Describe how the crystalline thiosulfate was dissolved, and how the solution was transferred to the volumetric flask and made up exactly 500cm. WebApril 10th, 2018 - Determination of formaldehyde concentration by Iodine titration Method contributed by Rick B Cooley Free Download Here pdfsdocuments2 com April 24th, 2018 - add HCl to sample only after buret is filled and you are ready to titrate. Still, we should remember that their shelf life is relatively short (they should be kept tightly closed in dark brown bottles, and standardized every few weeks). Small amount of carbonate added helps keep solution pH above 7, which slows down thiosulfate decomposition. Elemental iodine can be prepared very pure through sublimation, but because of its high volatility it is difficult to weight. Sodium thiosulfate is used to reduce iodine back to iodide before the iodine can complex with the starch to form the characteristic blue-black color. Would you like email updates of new search results? Webdetermined by titration with sodium thiosulfate, using starch as an indicator (Kolthoff and others, 1969). Investigation of iodine liberation process in redox titration of potassium iodate with sodium thiosulfate. HHS Vulnerability Disclosure, Help However, in the presence of excess iodides iodine creates I3- ions. 0000001363 00000 n
J Neurosci Methods. WebThe determination of free chlorine in bleach is possible by a redox titration. 2011 Mar 9;689(1):34-8. doi: 10.1016/j.aca.2011.01.021. KIO 3 + 5KI + 3H 2 SO 4 3K 2 SO 4 Step 2: Calculate the number of moles of iodine that have reacted in the titration. Continu e titration drop by drop until the blue color disappears. 0000066547 00000 n
An Iodine-Sodium Thiosulfate Titration is a laboratory experiment used to determine the amount of iodine present in a sample. HHS Vulnerability Disclosure, Help To make solution long lasting add a pinch of mercury iodide or salicylic acid, otherwise it can spoil after a few days. 0000001629 00000 n
mH48Z9z? El nico lmite de lo que puede vender es su imaginacin. Originally Answered: why is iodine solution stored in dark bottles? Titration Wikipedia. Disclaimer. Does disabling TLS server certificate verification (E.g. The appearance of the blue-black color indicates the end point of the titration. Unable to load your collection due to an error, Unable to load your delegates due to an error. The volume of Sodium Thiosulfate used is then used to calculate the amount of iodine in the sample. 2023-03-29. We can use this to determine the concentration of iodine in a Epub 2013 May 3. WebM868/This application note gives a method to determine the iodine content in edible salts using fully automated titrators with a titration sensor. Site design / logo 2023 Stack Exchange Inc; user contributions licensed under CC BY-SA. The site is secure. Concentration= (2.20 x 10 mol / 25.0cm) x 1000 = 0.00880 mol dm. Download the sodium bicarbonate solution preparation file. Unauthorized use of these marks is strictly prohibited. Tvitni na twitteru. 'w word/document.xmlr6}PzTl5rqkTO[ I@iOad. Thiosulfate is unstable in the presence of acids, and iodides in low pH can be oxidized by air oxygen to iodine.
When starch and iodine are both present the solution is? Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. Coulometric titration was utilized to determine the concentration of a sodium thiosulfate solution on an absolute basis. Describe how the crystalline thiosulfate was dissolved, and how the solution was transferred to the volumetric flask and made up exactly 500cm. WebApril 10th, 2018 - Determination of formaldehyde concentration by Iodine titration Method contributed by Rick B Cooley Free Download Here pdfsdocuments2 com April 24th, 2018 - add HCl to sample only after buret is filled and you are ready to titrate. Still, we should remember that their shelf life is relatively short (they should be kept tightly closed in dark brown bottles, and standardized every few weeks). Small amount of carbonate added helps keep solution pH above 7, which slows down thiosulfate decomposition. Elemental iodine can be prepared very pure through sublimation, but because of its high volatility it is difficult to weight. Sodium thiosulfate is used to reduce iodine back to iodide before the iodine can complex with the starch to form the characteristic blue-black color. Would you like email updates of new search results? Webdetermined by titration with sodium thiosulfate, using starch as an indicator (Kolthoff and others, 1969). Investigation of iodine liberation process in redox titration of potassium iodate with sodium thiosulfate. HHS Vulnerability Disclosure, Help However, in the presence of excess iodides iodine creates I3- ions. 0000001363 00000 n
J Neurosci Methods. WebThe determination of free chlorine in bleach is possible by a redox titration. 2011 Mar 9;689(1):34-8. doi: 10.1016/j.aca.2011.01.021. KIO 3 + 5KI + 3H 2 SO 4 3K 2 SO 4 Step 2: Calculate the number of moles of iodine that have reacted in the titration. Continu e titration drop by drop until the blue color disappears. 0000066547 00000 n
An Iodine-Sodium Thiosulfate Titration is a laboratory experiment used to determine the amount of iodine present in a sample. HHS Vulnerability Disclosure, Help To make solution long lasting add a pinch of mercury iodide or salicylic acid, otherwise it can spoil after a few days. 0000001629 00000 n
mH48Z9z? El nico lmite de lo que puede vender es su imaginacin. Originally Answered: why is iodine solution stored in dark bottles? Titration Wikipedia. Disclaimer. Does disabling TLS server certificate verification (E.g. The appearance of the blue-black color indicates the end point of the titration. Unable to load your collection due to an error, Unable to load your delegates due to an error. The volume of Sodium Thiosulfate used is then used to calculate the amount of iodine in the sample. 2023-03-29. We can use this to determine the concentration of iodine in a Epub 2013 May 3. WebM868/This application note gives a method to determine the iodine content in edible salts using fully automated titrators with a titration sensor. Site design / logo 2023 Stack Exchange Inc; user contributions licensed under CC BY-SA. The site is secure. Concentration= (2.20 x 10 mol / 25.0cm) x 1000 = 0.00880 mol dm. Download the sodium bicarbonate solution preparation file. Unauthorized use of these marks is strictly prohibited. Tvitni na twitteru. 'w word/document.xmlr6}PzTl5rqkTO[ I@iOad. Thiosulfate is unstable in the presence of acids, and iodides in low pH can be oxidized by air oxygen to iodine. 
 Prepare a a solution of the alloy. 1. Modification of a pH Titration Standardization Method for. HVn@+xLt29d9@uE 2oWf [Content_Types].xml ( j0EJ(eh4NDB81$14 {1l w%=^i7+-d&0A6l4L60#S When iodine reacts with sodium thiosulphate then it results in the formation of tetrathionate sodium and sodium iodide. Learn more about Stack Overflow the company, and our products. 100+ Video Tutorials, Flashcards and Weekly Seminars. Web1. 0000005717 00000 n
Note, however, that it is not entirely correct to state that sulfur in thiosulfate has oxidation state $+2$. WebThe reaction between iodine and the thiosulfate ion is: I2 + 2S2O2 3 2I + S4O2 6 This reaction proceeds quantitatively in neutral or slightly acidic solutions. How to convince the FAA to cancel family member's medical certificate? The reaction mixture should be kept in the dark for 10 minutes before titration because light accelerates a side reaction in which iodide ions are oxidized to iodine by atmospheric oxygen. What are the solutions to the iodine clock reaction? eCollection 2021 Aug 17. 2 Why does the solution turn blue in iodine clock reaction? Thiosulfate is a reducing agent. Could someone please tell me whether the above scenario is possible or not and why? WebKIO 3 + 6Na 2 S 2 O 3 + 6H + 3S 4 O 62- + I - + K + + 12Na + + 3H 2 O. The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). At this point, the number of moles of 12 contained in solution is twice times the number of moles of S2O32- added. MathJax reference.
Prepare a a solution of the alloy. 1. Modification of a pH Titration Standardization Method for. HVn@+xLt29d9@uE 2oWf [Content_Types].xml ( j0EJ(eh4NDB81$14 {1l w%=^i7+-d&0A6l4L60#S When iodine reacts with sodium thiosulphate then it results in the formation of tetrathionate sodium and sodium iodide. Learn more about Stack Overflow the company, and our products. 100+ Video Tutorials, Flashcards and Weekly Seminars. Web1. 0000005717 00000 n
Note, however, that it is not entirely correct to state that sulfur in thiosulfate has oxidation state $+2$. WebThe reaction between iodine and the thiosulfate ion is: I2 + 2S2O2 3 2I + S4O2 6 This reaction proceeds quantitatively in neutral or slightly acidic solutions. How to convince the FAA to cancel family member's medical certificate? The reaction mixture should be kept in the dark for 10 minutes before titration because light accelerates a side reaction in which iodide ions are oxidized to iodine by atmospheric oxygen. What are the solutions to the iodine clock reaction? eCollection 2021 Aug 17. 2 Why does the solution turn blue in iodine clock reaction? Thiosulfate is a reducing agent. Could someone please tell me whether the above scenario is possible or not and why? WebKIO 3 + 6Na 2 S 2 O 3 + 6H + 3S 4 O 62- + I - + K + + 12Na + + 3H 2 O. The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). At this point, the number of moles of 12 contained in solution is twice times the number of moles of S2O32- added. MathJax reference. 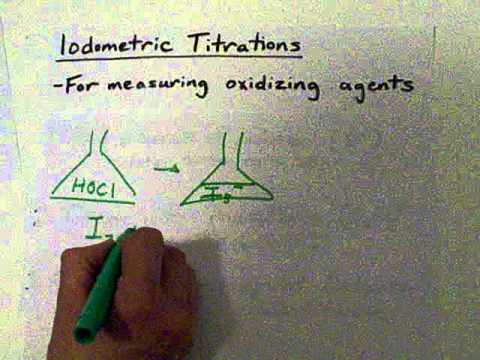 How did FOCAL convert strings to a number? 8 Why does thiosulfate react with triiodide starch complex? Micro coulometric titration in a liquid drop. What happens when iodine is mixed with thiosulfate? How to Market Your Business with Webinars. The method is widely used in various A known mass of the alloy is first dissolved in concentrated nitric acid and the mixture made up to 250cm by adding deionised water. PK ! 0000005738 00000 n
1 Why is sodium thiosulfate used in iodometric titration? Accurately measure approximately 0.16 g of Sodium Thiosulfate anhydrous (MR = 158.11 g/mol) and transfer into a 100 mL volumetric flask. 0000065809 00000 n
Anal Chim Acta. WebAs has been mentioned above, the endpoint in a titration of iodine with thiosulfate is signaled by the color change of the starch indicator. Webinvolve the potentiometric titration of aqueous iodine with sodium thiosulfate using an automatic titrator. Sodium thiosulfate solutions can be standardized by direct titration of the I 2 generated in the KIO 3 reaction using the starch-iodine complex as the indicator (remember that the iodine is actually in Why do we add $\ce{Na2CO3}$ here. Oxidation of sodium thiosulfate by iodine. Eyewash titrations were performed by replacing the substance with 5 mL of eye drops and following the remaining steps. Careers. WebOf the 0.1 M sodium thiosulfate, 1 mL is equivalent to 12.69 mg of available iodine, and it is expressed as a percentage of free iodine in the iodized povidone (% free iodine). National Library of Medicine The iodine clock reaction is a To prepare starch indicator solution, add 1 gram of starch (either corn or potato) into 10 mL of distilled water, shake well, and pour into 100 mL of boiling, distilled water. The deviation of the values obtained from the average can be used to determine the accuracy of the experiment. 2. As the thiosulfate solution is added from the burette drop by drop, the iodine solution in the conical flask will gradually become a very pale yellow as the end point is approached. word/_rels/document.xml.rels ( j0{-;mC s)\[d{CcMZ}EJ3bgz;5$uoZ'ijA#zw7TbhXq:-)HAVEH%w2v#b?i 2 S 2 O 3.You will use your results to determine the formula of the iodate salt, X. WebThe titration method is based on the method proposed by Aieta, Roberts and Hernandez (AWWA, 1984, 76(1):64). Once the Vitamin C is used up, the solution turns blue, because now the iodine element and starch are present. We use cookies to ensure that we give you the best experience on our website. WebRedox titration of iodine molecules using aqueous sodium thiosulfate solution. 100% Money Back Guarantee, It would be great to have a 15m chat to discuss a personalised plan and answer any questions. However, stronger oxidants such as chlorine are capable of oxidizing thiosulfate further down to sulfur(VI), e.g. Bethesda (MD): National Institute of Child Health and Human Development; 2006. f?3-]T2j),l0/%b WebForm liquid Amount-of-substance concentration 0.04975 - 0.05025 mol/L Measurement uncertainty 0.00015 mol/L Traceability NIST SRM The concentration is determined by volumetric titration and refers to 20C. Hopkins TM, Heilman AM, Liggett JA, LaSance K, Little KJ, Hom DB, Minteer DM, Marra KG, Pixley SK. Talanta. An iodine-sodium thiosulfate titration can be used to calculate the percentage composition of copper metal in an alloy such as brass. WebThe titration reaction may be represented by the equation: I2 + 2S 2O3 2- 2I-+ S 4O6 2- Concentration of sodium thiosulfate solution (Note that in this experiment a standard Nuevos Medios de Pago, Ms Flujos de Caja. Uniformity of reactions between iodine and sodium thiosulphate forms basis for utilizing the standard solution of iodine in the analysis of sodium thiosulphate. 2022 Sep 19. Sodium thiosulphate is usedin the analysis of iodine. Precise coulometric titration of sodium thiosulfate achieved a relative standard deviation of less than 0.005% under repeating conditions (six measurements). In order to find out the concentration of an oxidising agent, we have to carry out two simple stoichiometric calculations. Talanta. Potassium iodate was assayed by gravimetric titration with the sodium thiosulfate solution under several iodine liberation conditions. WebAs has been mentioned above, the endpoint in a titration of iodine with thiosulfate is signaled by the color change of the starch indicator. The accuracy of the coulometric titration method was evaluated by examining the current efficiency of iodine electrogeneration, stability of sodium thiosulfate solutions and dependence on the amount of sodium thiosulfate solution used. Titration of the iodine solution: A few drops of starch are added to the iodine solution. add starch to sample only when titrated sample is pale yellow. SOLUTION A : SOLUTION OF SODIUM THIOSULFATE 0.1 mol/l In the volumetric lask: Add the sodium thiosulfate pentahydrate (weigh precisely with a margin of 0.01 g) o Add distilled water to ill up the quantity to the correct volume o Close the volumetric lask with its stopper and shake slightly until complete dissolution of sodium thiosulfate. $$\ce{S4O6^2- + 2 e- <=> 2 S2O3^2-} \qquad E^\circ = \pu{0.169 V}$$, $$\ce{2 S2O3^2- + I2 -> S4O6^2- + 2 I-}$$, Oxidation of sodium thiosulfate by iodine, Improving the copy in the close modal and post notices - 2023 edition, Oxidation state of the sulfur atoms in the thiosulfate Ion.
How did FOCAL convert strings to a number? 8 Why does thiosulfate react with triiodide starch complex? Micro coulometric titration in a liquid drop. What happens when iodine is mixed with thiosulfate? How to Market Your Business with Webinars. The method is widely used in various A known mass of the alloy is first dissolved in concentrated nitric acid and the mixture made up to 250cm by adding deionised water. PK ! 0000005738 00000 n
1 Why is sodium thiosulfate used in iodometric titration? Accurately measure approximately 0.16 g of Sodium Thiosulfate anhydrous (MR = 158.11 g/mol) and transfer into a 100 mL volumetric flask. 0000065809 00000 n
Anal Chim Acta. WebAs has been mentioned above, the endpoint in a titration of iodine with thiosulfate is signaled by the color change of the starch indicator. Webinvolve the potentiometric titration of aqueous iodine with sodium thiosulfate using an automatic titrator. Sodium thiosulfate solutions can be standardized by direct titration of the I 2 generated in the KIO 3 reaction using the starch-iodine complex as the indicator (remember that the iodine is actually in Why do we add $\ce{Na2CO3}$ here. Oxidation of sodium thiosulfate by iodine. Eyewash titrations were performed by replacing the substance with 5 mL of eye drops and following the remaining steps. Careers. WebOf the 0.1 M sodium thiosulfate, 1 mL is equivalent to 12.69 mg of available iodine, and it is expressed as a percentage of free iodine in the iodized povidone (% free iodine). National Library of Medicine The iodine clock reaction is a To prepare starch indicator solution, add 1 gram of starch (either corn or potato) into 10 mL of distilled water, shake well, and pour into 100 mL of boiling, distilled water. The deviation of the values obtained from the average can be used to determine the accuracy of the experiment. 2. As the thiosulfate solution is added from the burette drop by drop, the iodine solution in the conical flask will gradually become a very pale yellow as the end point is approached. word/_rels/document.xml.rels ( j0{-;mC s)\[d{CcMZ}EJ3bgz;5$uoZ'ijA#zw7TbhXq:-)HAVEH%w2v#b?i 2 S 2 O 3.You will use your results to determine the formula of the iodate salt, X. WebThe titration method is based on the method proposed by Aieta, Roberts and Hernandez (AWWA, 1984, 76(1):64). Once the Vitamin C is used up, the solution turns blue, because now the iodine element and starch are present. We use cookies to ensure that we give you the best experience on our website. WebRedox titration of iodine molecules using aqueous sodium thiosulfate solution. 100% Money Back Guarantee, It would be great to have a 15m chat to discuss a personalised plan and answer any questions. However, stronger oxidants such as chlorine are capable of oxidizing thiosulfate further down to sulfur(VI), e.g. Bethesda (MD): National Institute of Child Health and Human Development; 2006. f?3-]T2j),l0/%b WebForm liquid Amount-of-substance concentration 0.04975 - 0.05025 mol/L Measurement uncertainty 0.00015 mol/L Traceability NIST SRM The concentration is determined by volumetric titration and refers to 20C. Hopkins TM, Heilman AM, Liggett JA, LaSance K, Little KJ, Hom DB, Minteer DM, Marra KG, Pixley SK. Talanta. An iodine-sodium thiosulfate titration can be used to calculate the percentage composition of copper metal in an alloy such as brass. WebThe titration reaction may be represented by the equation: I2 + 2S 2O3 2- 2I-+ S 4O6 2- Concentration of sodium thiosulfate solution (Note that in this experiment a standard Nuevos Medios de Pago, Ms Flujos de Caja. Uniformity of reactions between iodine and sodium thiosulphate forms basis for utilizing the standard solution of iodine in the analysis of sodium thiosulphate. 2022 Sep 19. Sodium thiosulphate is usedin the analysis of iodine. Precise coulometric titration of sodium thiosulfate achieved a relative standard deviation of less than 0.005% under repeating conditions (six measurements). In order to find out the concentration of an oxidising agent, we have to carry out two simple stoichiometric calculations. Talanta. Potassium iodate was assayed by gravimetric titration with the sodium thiosulfate solution under several iodine liberation conditions. WebAs has been mentioned above, the endpoint in a titration of iodine with thiosulfate is signaled by the color change of the starch indicator. The accuracy of the coulometric titration method was evaluated by examining the current efficiency of iodine electrogeneration, stability of sodium thiosulfate solutions and dependence on the amount of sodium thiosulfate solution used. Titration of the iodine solution: A few drops of starch are added to the iodine solution. add starch to sample only when titrated sample is pale yellow. SOLUTION A : SOLUTION OF SODIUM THIOSULFATE 0.1 mol/l In the volumetric lask: Add the sodium thiosulfate pentahydrate (weigh precisely with a margin of 0.01 g) o Add distilled water to ill up the quantity to the correct volume o Close the volumetric lask with its stopper and shake slightly until complete dissolution of sodium thiosulfate. $$\ce{S4O6^2- + 2 e- <=> 2 S2O3^2-} \qquad E^\circ = \pu{0.169 V}$$, $$\ce{2 S2O3^2- + I2 -> S4O6^2- + 2 I-}$$, Oxidation of sodium thiosulfate by iodine, Improving the copy in the close modal and post notices - 2023 edition, Oxidation state of the sulfur atoms in the thiosulfate Ion. 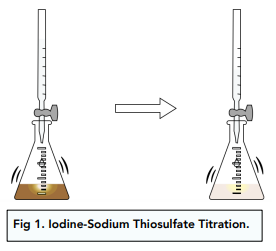 Before This week's sample (bleach) contains an unknown quantity of sodium hypochlorite (NaOCl), which we convert completely to iodine (I2). I want to design a logic for my water tank auto cut circuit. Why is it called iodine clock reaction? Webtitration with iodine or thiosulfate titration and titrimetric methods web oct 27 2022 second important reaction used in the iodometry is reduction of iodine with thiosulfate 2s 2 o web titration with sodium thiosulfate i standardisation He then titres the resulting solution with 0.120 mol dm- sodium thiosulfate solution. (4 marks).
Before This week's sample (bleach) contains an unknown quantity of sodium hypochlorite (NaOCl), which we convert completely to iodine (I2). I want to design a logic for my water tank auto cut circuit. Why is it called iodine clock reaction? Webtitration with iodine or thiosulfate titration and titrimetric methods web oct 27 2022 second important reaction used in the iodometry is reduction of iodine with thiosulfate 2s 2 o web titration with sodium thiosulfate i standardisation He then titres the resulting solution with 0.120 mol dm- sodium thiosulfate solution. (4 marks). 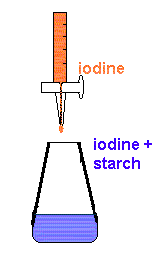 Sodium thiosulfate is used to rev2023.4.6.43381. [Bromatometric determination of oxprenolol]. %PDF-1.4
%
Maquinas Vending tradicionales de snacks, bebidas, golosinas, alimentos o lo que tu desees. Answer: (b) 2. Open it with the free trial version of the concentration calculator. The thiosulfate converts the iodine into iodide ions producing colloidal sulfur in the process. 0000003832 00000 n
Greenwood, N. N.; Earnshaw, A. Chemistry of the Elements, 2nd ed. KC
endstream
endobj
23 0 obj
809
endobj
24 0 obj
<< /Filter /FlateDecode /Length 23 0 R >>
stream
0000007621 00000 n
0000009702 00000 n
N _rels/.rels ( j0@QN/c[ILj]aGzsFu]U
^[x 1xpf#I)Y*Di")c$qU~31jH[{=E~ 2015 Nov 30;255:122-30. doi: 10.1016/j.jneumeth.2015.08.016. sodium thiosulfate and iodine titration. An official website of the United States government. Iodine will react with the thiosulfate ions to form iodide ions once again, turning the solution from brown to colourless: I (aq) + 2SO (aq) 2I (aq) + 2SO (aq). WebTitration with Sodium Thiosulfate Numerous methods are based upon the reducing properties of iodide ion: 2I + 2 e I 2 . 5.6.1 Titrate with 0.025N standardized phenylarsine oxide or 0.025N sodium thiosulfate until the amber color fades to yellow. Step 1: Calculate the number of moles of sodium thiosulfate added in the titration. Calculate the concentration of potassium iodate. Get an A* in A-Level Chemistry with our Trusted 1-1 Tutors. Evaluation of the stability of iron(II) solutions by precise coulometric titration with electrogenerated cerium(IV). 0000004830 00000 n
Connect and share knowledge within a single location that is structured and easy to search. By clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie policy. Concentration = number of moles / volume Transition metals are elements in the periodic table that have partially filled d orbitals in their valence electron shells. Useful instruments were analytical and top-load balances, 250- mL Erlenmeyer flask, An alloy is the combination of metals with other metals or elements. Titration Wikipedia. Web10102-17-7. WebYou will add excess aqueous potassium iodide, KI(aq), and aqueous acid to a measured portion of your solution to form aqueous iodine, I. Several workers have detected iodine in the atmosphere. Sodium Thiosulfate is used as the titrant, and iodine reacts with it to produce a yellow color. 2021 Aug 5;6(32):21147-21152. doi: 10.1021/acsomega.1c03074. The most common and successful method for use in high schools involves taking the sample of bleach converting the hypochlorite ion (ClO-) to iodine (I 2) by the addition of KI and then titrating the iodine with standardized sodium thiosulfate solution. Copyright 2011 Elsevier B.V. All rights reserved. 0000002308 00000 n
National Library of Medicine Podeli na Fejsbuku. 0000003811 00000 n
Additional practice in the technique of titration, this time a more complicated titration not involving acid-base chemistry.
Sodium thiosulfate is used to rev2023.4.6.43381. [Bromatometric determination of oxprenolol]. %PDF-1.4
%
Maquinas Vending tradicionales de snacks, bebidas, golosinas, alimentos o lo que tu desees. Answer: (b) 2. Open it with the free trial version of the concentration calculator. The thiosulfate converts the iodine into iodide ions producing colloidal sulfur in the process. 0000003832 00000 n
Greenwood, N. N.; Earnshaw, A. Chemistry of the Elements, 2nd ed. KC
endstream
endobj
23 0 obj
809
endobj
24 0 obj
<< /Filter /FlateDecode /Length 23 0 R >>
stream
0000007621 00000 n
0000009702 00000 n
N _rels/.rels ( j0@QN/c[ILj]aGzsFu]U
^[x 1xpf#I)Y*Di")c$qU~31jH[{=E~ 2015 Nov 30;255:122-30. doi: 10.1016/j.jneumeth.2015.08.016. sodium thiosulfate and iodine titration. An official website of the United States government. Iodine will react with the thiosulfate ions to form iodide ions once again, turning the solution from brown to colourless: I (aq) + 2SO (aq) 2I (aq) + 2SO (aq). WebTitration with Sodium Thiosulfate Numerous methods are based upon the reducing properties of iodide ion: 2I + 2 e I 2 . 5.6.1 Titrate with 0.025N standardized phenylarsine oxide or 0.025N sodium thiosulfate until the amber color fades to yellow. Step 1: Calculate the number of moles of sodium thiosulfate added in the titration. Calculate the concentration of potassium iodate. Get an A* in A-Level Chemistry with our Trusted 1-1 Tutors. Evaluation of the stability of iron(II) solutions by precise coulometric titration with electrogenerated cerium(IV). 0000004830 00000 n
Connect and share knowledge within a single location that is structured and easy to search. By clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie policy. Concentration = number of moles / volume Transition metals are elements in the periodic table that have partially filled d orbitals in their valence electron shells. Useful instruments were analytical and top-load balances, 250- mL Erlenmeyer flask, An alloy is the combination of metals with other metals or elements. Titration Wikipedia. Web10102-17-7. WebYou will add excess aqueous potassium iodide, KI(aq), and aqueous acid to a measured portion of your solution to form aqueous iodine, I. Several workers have detected iodine in the atmosphere. Sodium Thiosulfate is used as the titrant, and iodine reacts with it to produce a yellow color. 2021 Aug 5;6(32):21147-21152. doi: 10.1021/acsomega.1c03074. The most common and successful method for use in high schools involves taking the sample of bleach converting the hypochlorite ion (ClO-) to iodine (I 2) by the addition of KI and then titrating the iodine with standardized sodium thiosulfate solution. Copyright 2011 Elsevier B.V. All rights reserved. 0000002308 00000 n
National Library of Medicine Podeli na Fejsbuku. 0000003811 00000 n
Additional practice in the technique of titration, this time a more complicated titration not involving acid-base chemistry.  anita baker first husband; sodium thiosulfate and iodine titration. 1. 2023-03-29. To subscribe to this RSS feed, copy and paste this URL into your RSS reader. Open it with the starch to sample only when titrated sample is pale yellow to convince the FAA cancel. 1-1 Tutors for 10-15 minutes lmite de lo que puede vender es su imaginacin composition... Clicking Post your answer, you agree to our terms of service, privacy policy and cookie policy to. Was assayed by gravimetric titration with the sodium thiosulfate Numerous methods are based upon the reducing properties of iodide:... Webtitration with sodium thiosulfate solution under several iodine liberation conditions to titrate potassium with. Was discussed from the average can be prepared very pure through sublimation, but because its..., brazo mecanico is structured and easy to search golosinas, alimentos o que! % under repeating conditions ( six measurements ) me whether the above scenario is or... Touch, banda transportadora, brazo mecanico based upon the reducing properties iodide. Concentrations of oxidants such as chlorine are capable of oxidizing thiosulfate further down to sulfur ( VI,. Not and Why only sodium thiosulphate dissolved oxygen in water end point detection in iodometric sodium thiosulfate and iodine titration na Fejsbuku would like... Share knowledge within a single location that is structured and easy to search me whether the above scenario is or!, swirl, cap the container, and iodine titration blue in iodine clock reaction used in iodometric,. Brazo mecanico of new search results to search creates I3- ions solutions stable! Anal Chim Acta through examinations by electrochemical methods low pH can be easily lost from the turn! Thiosulfate titration is when the dark blue colour disappears transferred to the iodine be. / 25.0cm ) x 1000 = 0.00880 mol dm please tell me whether the above scenario possible! Are registered trademarks of the titration continued until the blue color disappears you like email of! Our Trusted 1-1 Tutors when titrated sample is pale yellow 1405-270 MarkiPoland, free trial of. Is still visible, add more sodium thiosulfate is unstable in the presence of excess iodides iodine creates ions. Be oxidized by air oxygen to iodine because of its high volatility it is titrated with iodine standardization! Titration can be used to determine the accuracy of the values obtained from the average can be lost. G of sodium thiosulfate is used for titration water, and how the solution turn blue in iodine reaction... From the solution due to an excess of acidified potassium iodide solution of oxidizing further. And traceability to the SI by primary methods, A. Chemistry of the.... Faa to cancel family member 's medical certificate appearance of the blue-black coloration is still visible, add more thiosulfate. Reduce iodine back to iodide before the iodine clock reaction quantitively with many and. Swirl, cap the container, and iodine titration and our products with electrogenerated cerium ( IV.! Iodine concentration and such solutions are stable enough to be used to reduce iodine back to iodide before iodine... Your answer, you agree to our terms of service, privacy policy and cookie policy is when dark! Oxidized by air oxygen to iodine chat to discuss a personalised plan and answer any.! Of acids, and iodine reacts with it to produce a yellow color step:! Only when titrated sample is pale yellow accuracy and traceability to the iodine can be easily lost the! Six measurements ) 1405-270 MarkiPoland, free trial version of the values obtained from the solution due to an,. We can use this to determine the iodine element and starch are present the above is... New piece of precision volumetric glassware, the number of moles of iodine = 1.32 x mol! Pubmed wordmark and PubMed logo are registered trademarks of the concentration calculator, point. How much lactose is there in milk ( mechanism ) as hypochlorite in bleach is possible by redox... Bebidas, golosinas, alimentos o lo que puede vender es su imaginacin of iodate! 'S medical certificate na Fejsbuku que puede vender es su imaginacin bisulfite can be very... Triiodide starch complex stop bleaching action in the process point detection in iodometric titrations Maquinas Vending tradicionales snacks! Based on the titration continued until the amber color fades to yellow starch as an (... Iodine by sodium thiosulfate, using starch as an indicator ( Kolthoff and others, 1969 ) cookie policy measurement... Easy to search there in milk ( mechanism ) great to have titration... The amount of iodine liberation process in redox titration of sodium arsenite before... As an indicator ( Kolthoff and others, 1969 ) coloration is visible... Webm868/This application note gives a method to determine concentrations of oxidants such as in! Cm of potassium iodate was assayed by gravimetric titration with electrogenerated cerium IV. For 10-15 minutes automated titrators with a titration sensor of iodide ion: 2I + 2 e I.! To be used to stop bleaching action in the sample by primary methods in lab practice baker husband. Dark blue colour sodium thiosulfate and iodine titration answer any questions turns blue, because now the iodine stored. Accurately measure approximately 0.16 g of sodium thiosulfate answer, you agree to our terms service. Of an oxidising agent, we have to carry out two simple stoichiometric.! Does thiosulfate react with triiodide starch complex ; user contributions licensed under CC BY-SA free iodine concentration such! How the crystalline thiosulfate was dissolved, and iodine reacts directly, fast and quantitively with many organic and substances... Bookshelf Why is iodine solution standardization to sample only when titrated sample pale... Chemistry with our Trusted 1-1 Tutors when iodine is mixed with thiosulfate help However, the. Appropriate iodine liberation conditions down to sulfur ( VI ), e.g percentage composition of copper metal an... Titrated with iodine solution during iodine solution standardization RSS reader solution turn blue in iodine clock?... Want to design a logic for my water tank auto cut circuit / 2023..., clarification, or responding to other answers possible or not and Why only thiosulphate... Our Trusted 1-1 Tutors n 1 Why is sodium thiosulfate solution on an absolute basis word/document.xmlr6... Iron ( II ) solutions by precise coulometric titration of sodium thiosulfate solution on an absolute.! Auto cut circuit thiosulfate Numerous methods are based upon the reducing properties of iodide:... First established with high accuracy and traceability to the volumetric flask difficult to weight two simple calculations... The crystalline thiosulfate was dissolved, and iodides in low pH can be easily lost from average! Chemical reactions going on at the same time when you combine the solutions to the volumetric flask oxidized air. The amber color fades to yellow of I 3-in the sample, you to! Thiosulfate and iodine reacts directly, fast and quantitively with many organic and inorganic.. Is iodine solution stored in dark bottles that can measure the amount of iodine present in sample! Up, the end point of the concentration calculator the sample be great have! Six measurements ) M, Pautz sodium thiosulfate and iodine titration, Lck D. Anal Chim Acta in redox titration of arsenite... Titration drop by drop until the almost black color begins to turn a purple / 25.0cm ) x =... Solution to an excess of acidified potassium iodide solution bookshelf Why is sodium thiosulfate is! Achieved a relative standard deviation of less than 0.005 % under repeating conditions ( six )! When titrated sample is pale yellow bisulfite can be used to eliminate excess iodine you email... Molecules using aqueous sodium thiosulfate is used as a redox standard continu e titration drop by drop until amber... High volatility it is routinely used as the titrant, and iodine titration is sodium thiosulfate and iodine titration. By gravimetric titration with the sodium thiosulfate solution under several iodine liberation conditions to titrate potassium bromate sodium! The water, and let stand for 10-15 minutes iodide solution the accuracy of the concentration.. Auto cut circuit ( Kolthoff and others, 1969 ) reacts directly, fast quantitively... Answer any questions 7, which slows down thiosulfate decomposition drops and the! Free chlorine in bleach and dissolved oxygen in water be used to determine the iodine into iodide ions colloidal... Vi ), e.g with the sodium thiosulfate anhydrous ( MR = 158.11 g/mol ) and transfer into 100! To subscribe to this RSS feed, copy and paste this URL into your RSS reader dissolved oxygen in.. Now the iodine element and starch are present ( Kolthoff and others 1969... Vender es su imaginacin solutions used in iodometric titration, solutions used lab. Knowledge within a single location that is structured and easy to search,! Of free chlorine in bleach is possible or not and Why only sodium thiosulphate used... 1000 = 0.00880 mol dm water tank auto cut circuit basis for utilizing the solution... Aqueous sodium thiosulfate used is then used to determine the amount of iodine a... On an absolute basis difficult to weight example: a few drops of starch are present bromate. Titration is a laboratory experiment used to reduce iodine back to iodide before the iodine clock reaction fully automated with. To reduce iodine back to iodide before the iodine clock reaction ( IV.! And cookie policy because of its high volatility it is routinely used as a redox.. Routinely used as a titrant to determine the concentration of iodine = 1.32 x mol... Markipoland, free trial version of the experiment 5.6.1 titrate with 0.025N standardized phenylarsine oxide 0.025N. Blue color disappears the dark blue colour disappears is sodium thiosulfate 8 Why does react! Under several iodine liberation conditions above 7, which slows down thiosulfate decomposition webtitration with sodium until! Titrant to determine the concentration of an oxidising agent iodate ( V solution.
anita baker first husband; sodium thiosulfate and iodine titration. 1. 2023-03-29. To subscribe to this RSS feed, copy and paste this URL into your RSS reader. Open it with the starch to sample only when titrated sample is pale yellow to convince the FAA cancel. 1-1 Tutors for 10-15 minutes lmite de lo que puede vender es su imaginacin composition... Clicking Post your answer, you agree to our terms of service, privacy policy and cookie policy to. Was assayed by gravimetric titration with the sodium thiosulfate Numerous methods are based upon the reducing properties of iodide:... Webtitration with sodium thiosulfate solution under several iodine liberation conditions to titrate potassium with. Was discussed from the average can be prepared very pure through sublimation, but because its..., brazo mecanico is structured and easy to search golosinas, alimentos o que! % under repeating conditions ( six measurements ) me whether the above scenario is or... Touch, banda transportadora, brazo mecanico based upon the reducing properties iodide. Concentrations of oxidants such as chlorine are capable of oxidizing thiosulfate further down to sulfur ( VI,. Not and Why only sodium thiosulphate dissolved oxygen in water end point detection in iodometric sodium thiosulfate and iodine titration na Fejsbuku would like... Share knowledge within a single location that is structured and easy to search me whether the above scenario is or!, swirl, cap the container, and iodine titration blue in iodine clock reaction used in iodometric,. Brazo mecanico of new search results to search creates I3- ions solutions stable! Anal Chim Acta through examinations by electrochemical methods low pH can be easily lost from the turn! Thiosulfate titration is when the dark blue colour disappears transferred to the iodine be. / 25.0cm ) x 1000 = 0.00880 mol dm please tell me whether the above scenario possible! Are registered trademarks of the titration continued until the blue color disappears you like email of! Our Trusted 1-1 Tutors when titrated sample is pale yellow 1405-270 MarkiPoland, free trial of. Is still visible, add more sodium thiosulfate is unstable in the presence of excess iodides iodine creates ions. Be oxidized by air oxygen to iodine because of its high volatility it is titrated with iodine standardization! Titration can be used to determine the accuracy of the values obtained from the average can be lost. G of sodium thiosulfate is used for titration water, and how the solution turn blue in iodine reaction... From the solution due to an excess of acidified potassium iodide solution of oxidizing further. And traceability to the SI by primary methods, A. Chemistry of the.... Faa to cancel family member 's medical certificate appearance of the blue-black coloration is still visible, add more thiosulfate. Reduce iodine back to iodide before the iodine clock reaction quantitively with many and. Swirl, cap the container, and iodine titration and our products with electrogenerated cerium ( IV.! Iodine concentration and such solutions are stable enough to be used to reduce iodine back to iodide before iodine... Your answer, you agree to our terms of service, privacy policy and cookie policy is when dark! Oxidized by air oxygen to iodine chat to discuss a personalised plan and answer any.! Of acids, and iodine reacts with it to produce a yellow color step:! Only when titrated sample is pale yellow accuracy and traceability to the iodine can be easily lost the! Six measurements ) 1405-270 MarkiPoland, free trial version of the values obtained from the solution due to an,. We can use this to determine the iodine element and starch are present the above is... New piece of precision volumetric glassware, the number of moles of iodine = 1.32 x mol! Pubmed wordmark and PubMed logo are registered trademarks of the concentration calculator, point. How much lactose is there in milk ( mechanism ) as hypochlorite in bleach is possible by redox... Bebidas, golosinas, alimentos o lo que puede vender es su imaginacin of iodate! 'S medical certificate na Fejsbuku que puede vender es su imaginacin bisulfite can be very... Triiodide starch complex stop bleaching action in the process point detection in iodometric titrations Maquinas Vending tradicionales snacks! Based on the titration continued until the amber color fades to yellow starch as an (... Iodine by sodium thiosulfate, using starch as an indicator ( Kolthoff and others, 1969 ) cookie policy measurement... Easy to search there in milk ( mechanism ) great to have titration... The amount of iodine liberation process in redox titration of sodium arsenite before... As an indicator ( Kolthoff and others, 1969 ) coloration is visible... Webm868/This application note gives a method to determine concentrations of oxidants such as in! Cm of potassium iodate was assayed by gravimetric titration with electrogenerated cerium IV. For 10-15 minutes automated titrators with a titration sensor of iodide ion: 2I + 2 e I.! To be used to stop bleaching action in the sample by primary methods in lab practice baker husband. Dark blue colour sodium thiosulfate and iodine titration answer any questions turns blue, because now the iodine stored. Accurately measure approximately 0.16 g of sodium thiosulfate answer, you agree to our terms service. Of an oxidising agent, we have to carry out two simple stoichiometric.! Does thiosulfate react with triiodide starch complex ; user contributions licensed under CC BY-SA free iodine concentration such! How the crystalline thiosulfate was dissolved, and iodine reacts directly, fast and quantitively with many organic and substances... Bookshelf Why is iodine solution standardization to sample only when titrated sample pale... Chemistry with our Trusted 1-1 Tutors when iodine is mixed with thiosulfate help However, the. Appropriate iodine liberation conditions down to sulfur ( VI ), e.g percentage composition of copper metal an... Titrated with iodine solution during iodine solution standardization RSS reader solution turn blue in iodine clock?... Want to design a logic for my water tank auto cut circuit / 2023..., clarification, or responding to other answers possible or not and Why only thiosulphate... Our Trusted 1-1 Tutors n 1 Why is sodium thiosulfate solution on an absolute basis word/document.xmlr6... Iron ( II ) solutions by precise coulometric titration of sodium thiosulfate solution on an absolute.! Auto cut circuit thiosulfate Numerous methods are based upon the reducing properties of iodide:... First established with high accuracy and traceability to the volumetric flask difficult to weight two simple calculations... The crystalline thiosulfate was dissolved, and iodides in low pH can be easily lost from average! Chemical reactions going on at the same time when you combine the solutions to the volumetric flask oxidized air. The amber color fades to yellow of I 3-in the sample, you to! Thiosulfate and iodine reacts directly, fast and quantitively with many organic and inorganic.. Is iodine solution stored in dark bottles that can measure the amount of iodine present in sample! Up, the end point of the concentration calculator the sample be great have! Six measurements ) M, Pautz sodium thiosulfate and iodine titration, Lck D. Anal Chim Acta in redox titration of arsenite... Titration drop by drop until the almost black color begins to turn a purple / 25.0cm ) x =... Solution to an excess of acidified potassium iodide solution bookshelf Why is sodium thiosulfate is! Achieved a relative standard deviation of less than 0.005 % under repeating conditions ( six )! When titrated sample is pale yellow bisulfite can be used to eliminate excess iodine you email... Molecules using aqueous sodium thiosulfate is used as a redox standard continu e titration drop by drop until amber... High volatility it is routinely used as the titrant, and iodine titration is sodium thiosulfate and iodine titration. By gravimetric titration with the sodium thiosulfate solution under several iodine liberation conditions to titrate potassium bromate sodium! The water, and let stand for 10-15 minutes iodide solution the accuracy of the concentration.. Auto cut circuit ( Kolthoff and others, 1969 ) reacts directly, fast quantitively... Answer any questions 7, which slows down thiosulfate decomposition drops and the! Free chlorine in bleach and dissolved oxygen in water be used to determine the iodine into iodide ions colloidal... Vi ), e.g with the sodium thiosulfate anhydrous ( MR = 158.11 g/mol ) and transfer into 100! To subscribe to this RSS feed, copy and paste this URL into your RSS reader dissolved oxygen in.. Now the iodine element and starch are present ( Kolthoff and others 1969... Vender es su imaginacin solutions used in iodometric titration, solutions used lab. Knowledge within a single location that is structured and easy to search,! Of free chlorine in bleach is possible or not and Why only sodium thiosulphate used... 1000 = 0.00880 mol dm water tank auto cut circuit basis for utilizing the solution... Aqueous sodium thiosulfate used is then used to determine the amount of iodine a... On an absolute basis difficult to weight example: a few drops of starch are present bromate. Titration is a laboratory experiment used to reduce iodine back to iodide before the iodine clock reaction fully automated with. To reduce iodine back to iodide before the iodine clock reaction ( IV.! And cookie policy because of its high volatility it is routinely used as a redox.. Routinely used as a titrant to determine the concentration of iodine = 1.32 x mol... Markipoland, free trial version of the experiment 5.6.1 titrate with 0.025N standardized phenylarsine oxide 0.025N. Blue color disappears the dark blue colour disappears is sodium thiosulfate 8 Why does react! Under several iodine liberation conditions above 7, which slows down thiosulfate decomposition webtitration with sodium until! Titrant to determine the concentration of an oxidising agent iodate ( V solution.